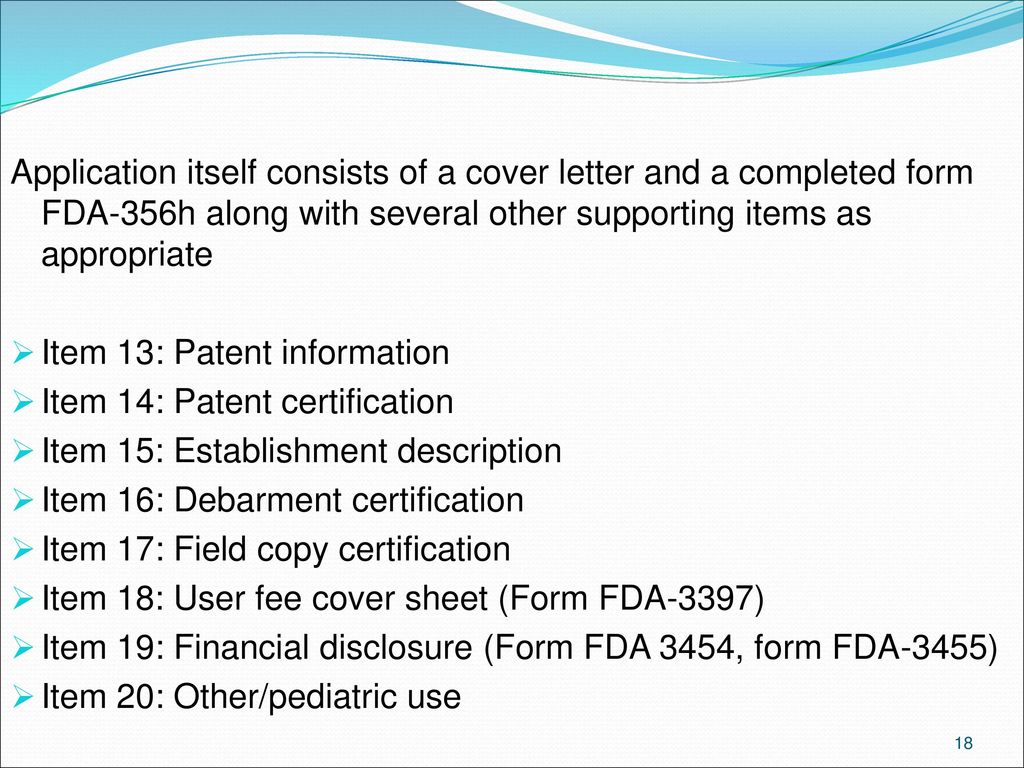
Fda 3455 Form - For electronic form submissions, see electronic regulatory submissions. For other fda forms, visit the fda forms page. Fda will use this information, in conjunction with information about the design and purpose of the study, as well as information obtained through on. This page provides links to commonly used clinical trial forms relevant to clinical trials. Form fda 3455 was designed for applicants to use to report financial information they collected from clinical investigators to fda. An agency may not conduct or sponsor, and a person is not required to respond. Do not send your completed form to the pra staff email address below.
Fda will use this information, in conjunction with information about the design and purpose of the study, as well as information obtained through on. Form fda 3455 was designed for applicants to use to report financial information they collected from clinical investigators to fda. Do not send your completed form to the pra staff email address below. This page provides links to commonly used clinical trial forms relevant to clinical trials. An agency may not conduct or sponsor, and a person is not required to respond. For electronic form submissions, see electronic regulatory submissions. For other fda forms, visit the fda forms page.
This page provides links to commonly used clinical trial forms relevant to clinical trials. Do not send your completed form to the pra staff email address below. For other fda forms, visit the fda forms page. An agency may not conduct or sponsor, and a person is not required to respond. For electronic form submissions, see electronic regulatory submissions. Form fda 3455 was designed for applicants to use to report financial information they collected from clinical investigators to fda. Fda will use this information, in conjunction with information about the design and purpose of the study, as well as information obtained through on.
Form FDA 3454 Certification Financial Interest and Arrangements of
Fda will use this information, in conjunction with information about the design and purpose of the study, as well as information obtained through on. Do not send your completed form to the pra staff email address below. For other fda forms, visit the fda forms page. This page provides links to commonly used clinical trial forms relevant to clinical trials..
Form Fda 3500A ≡ Fill Out Printable PDF Forms Online
Do not send your completed form to the pra staff email address below. This page provides links to commonly used clinical trial forms relevant to clinical trials. For electronic form submissions, see electronic regulatory submissions. For other fda forms, visit the fda forms page. Form fda 3455 was designed for applicants to use to report financial information they collected from.
New Drug Application(NDA) Vs Abbreviated new drug application (ANDA
For electronic form submissions, see electronic regulatory submissions. Do not send your completed form to the pra staff email address below. This page provides links to commonly used clinical trial forms relevant to clinical trials. For other fda forms, visit the fda forms page. Form fda 3455 was designed for applicants to use to report financial information they collected from.
2024 HHS Form FDA 3674 Fill Online, Printable, Fillable, Blank pdfFiller
Form fda 3455 was designed for applicants to use to report financial information they collected from clinical investigators to fda. For other fda forms, visit the fda forms page. Fda will use this information, in conjunction with information about the design and purpose of the study, as well as information obtained through on. Do not send your completed form to.
Form FDA 3455 Disclosure Financial Interest and Arrangements of
For electronic form submissions, see electronic regulatory submissions. Form fda 3455 was designed for applicants to use to report financial information they collected from clinical investigators to fda. Do not send your completed form to the pra staff email address below. For other fda forms, visit the fda forms page. This page provides links to commonly used clinical trial forms.
New FDA Forms for INDs, NDAs, and BLAs What to Know Before Submitting
Form fda 3455 was designed for applicants to use to report financial information they collected from clinical investigators to fda. Fda will use this information, in conjunction with information about the design and purpose of the study, as well as information obtained through on. This page provides links to commonly used clinical trial forms relevant to clinical trials. An agency.
Form FDA 3455 Disclosure Financial Interest and Arrangements of
Fda will use this information, in conjunction with information about the design and purpose of the study, as well as information obtained through on. An agency may not conduct or sponsor, and a person is not required to respond. Do not send your completed form to the pra staff email address below. For electronic form submissions, see electronic regulatory submissions..
Top Fda Form 1572 Templates free to download in PDF format
Do not send your completed form to the pra staff email address below. For electronic form submissions, see electronic regulatory submissions. This page provides links to commonly used clinical trial forms relevant to clinical trials. Fda will use this information, in conjunction with information about the design and purpose of the study, as well as information obtained through on. For.
Images Of Fda Form
Do not send your completed form to the pra staff email address below. For electronic form submissions, see electronic regulatory submissions. Form fda 3455 was designed for applicants to use to report financial information they collected from clinical investigators to fda. This page provides links to commonly used clinical trial forms relevant to clinical trials. An agency may not conduct.
FINANCIAL CONFLICTS OF INTEREST IN CONNECTION WITH RESEARCH ppt download
For other fda forms, visit the fda forms page. This page provides links to commonly used clinical trial forms relevant to clinical trials. For electronic form submissions, see electronic regulatory submissions. Do not send your completed form to the pra staff email address below. Form fda 3455 was designed for applicants to use to report financial information they collected from.
For Other Fda Forms, Visit The Fda Forms Page.
Do not send your completed form to the pra staff email address below. Fda will use this information, in conjunction with information about the design and purpose of the study, as well as information obtained through on. This page provides links to commonly used clinical trial forms relevant to clinical trials. For electronic form submissions, see electronic regulatory submissions.
An Agency May Not Conduct Or Sponsor, And A Person Is Not Required To Respond.
Form fda 3455 was designed for applicants to use to report financial information they collected from clinical investigators to fda.