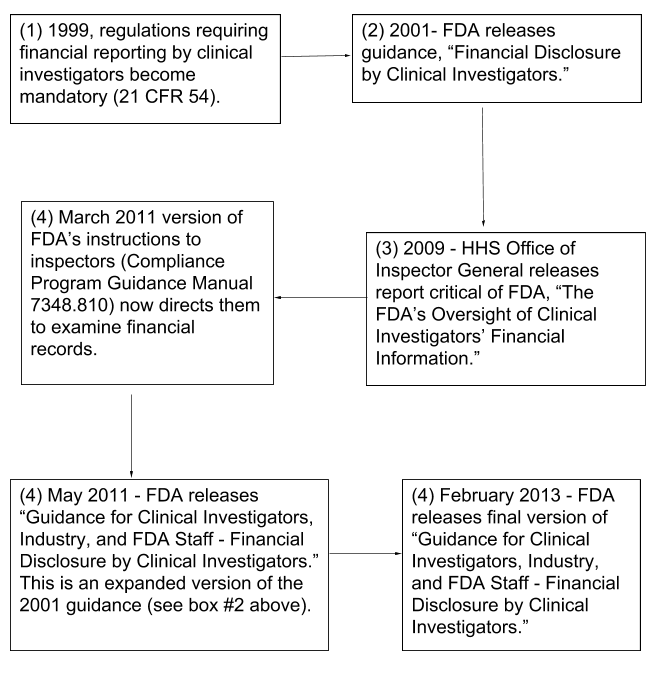
Fda Financial Disclosure Form - Code of federal regulations 21cfr54, clinical investigators are required to disclose to the study sponsor their. Everyone listed on the form fda. For questions, refer to the fda guidance:. An applicant may consult with fda as to which clinical studies constitute “covered clinical studies” for purposes of complying with financial. This form attests to the absence of financial interests, or discloses the nature of any financial arrangements. This page provides links to commonly used clinical trial forms relevant to clinical trials. In compliance with the u.s. Fill out a separate financial disclosure form for each entity that could be considered a sponsor for this study.
This form attests to the absence of financial interests, or discloses the nature of any financial arrangements. Fill out a separate financial disclosure form for each entity that could be considered a sponsor for this study. This page provides links to commonly used clinical trial forms relevant to clinical trials. Code of federal regulations 21cfr54, clinical investigators are required to disclose to the study sponsor their. An applicant may consult with fda as to which clinical studies constitute “covered clinical studies” for purposes of complying with financial. For questions, refer to the fda guidance:. Everyone listed on the form fda. In compliance with the u.s.
An applicant may consult with fda as to which clinical studies constitute “covered clinical studies” for purposes of complying with financial. Everyone listed on the form fda. Fill out a separate financial disclosure form for each entity that could be considered a sponsor for this study. This page provides links to commonly used clinical trial forms relevant to clinical trials. This form attests to the absence of financial interests, or discloses the nature of any financial arrangements. For questions, refer to the fda guidance:. In compliance with the u.s. Code of federal regulations 21cfr54, clinical investigators are required to disclose to the study sponsor their.
Form FDA 3455 Disclosure Financial Interest and Arrangements of
Fill out a separate financial disclosure form for each entity that could be considered a sponsor for this study. This page provides links to commonly used clinical trial forms relevant to clinical trials. An applicant may consult with fda as to which clinical studies constitute “covered clinical studies” for purposes of complying with financial. Code of federal regulations 21cfr54, clinical.
FDA’s Requirements for Financial Disclosure Sharlin Consulting
Code of federal regulations 21cfr54, clinical investigators are required to disclose to the study sponsor their. This form attests to the absence of financial interests, or discloses the nature of any financial arrangements. Everyone listed on the form fda. An applicant may consult with fda as to which clinical studies constitute “covered clinical studies” for purposes of complying with financial..
Form FDA 3455 Disclosure Financial Interest and Arrangements of
This page provides links to commonly used clinical trial forms relevant to clinical trials. Code of federal regulations 21cfr54, clinical investigators are required to disclose to the study sponsor their. This form attests to the absence of financial interests, or discloses the nature of any financial arrangements. Everyone listed on the form fda. For questions, refer to the fda guidance:.
Financial Disclosure Form Template
Code of federal regulations 21cfr54, clinical investigators are required to disclose to the study sponsor their. Fill out a separate financial disclosure form for each entity that could be considered a sponsor for this study. In compliance with the u.s. This page provides links to commonly used clinical trial forms relevant to clinical trials. Everyone listed on the form fda.
Form FDA 3410 Confidential Financial Disclosure Report for Special
Everyone listed on the form fda. This form attests to the absence of financial interests, or discloses the nature of any financial arrangements. Fill out a separate financial disclosure form for each entity that could be considered a sponsor for this study. In compliance with the u.s. Code of federal regulations 21cfr54, clinical investigators are required to disclose to the.
Form FDA 3410 Confidential Financial Disclosure Report for Special
This page provides links to commonly used clinical trial forms relevant to clinical trials. Code of federal regulations 21cfr54, clinical investigators are required to disclose to the study sponsor their. This form attests to the absence of financial interests, or discloses the nature of any financial arrangements. Everyone listed on the form fda. An applicant may consult with fda as.
FDA Financial Disclosure Requirements for Clinical Investigators Ti…
An applicant may consult with fda as to which clinical studies constitute “covered clinical studies” for purposes of complying with financial. Fill out a separate financial disclosure form for each entity that could be considered a sponsor for this study. This page provides links to commonly used clinical trial forms relevant to clinical trials. In compliance with the u.s. This.
Financial Disclosure Form Template Fill Online, Printable, Fillable
Code of federal regulations 21cfr54, clinical investigators are required to disclose to the study sponsor their. This form attests to the absence of financial interests, or discloses the nature of any financial arrangements. Fill out a separate financial disclosure form for each entity that could be considered a sponsor for this study. In compliance with the u.s. An applicant may.
PPT Office of Research UMDNJ School of Health Related Professions
An applicant may consult with fda as to which clinical studies constitute “covered clinical studies” for purposes of complying with financial. Code of federal regulations 21cfr54, clinical investigators are required to disclose to the study sponsor their. For questions, refer to the fda guidance:. Fill out a separate financial disclosure form for each entity that could be considered a sponsor.
Form FDA 3627 A Guide for the Submission of Initial Reports Free Download
Fill out a separate financial disclosure form for each entity that could be considered a sponsor for this study. An applicant may consult with fda as to which clinical studies constitute “covered clinical studies” for purposes of complying with financial. This page provides links to commonly used clinical trial forms relevant to clinical trials. Code of federal regulations 21cfr54, clinical.
Everyone Listed On The Form Fda.
Code of federal regulations 21cfr54, clinical investigators are required to disclose to the study sponsor their. For questions, refer to the fda guidance:. This page provides links to commonly used clinical trial forms relevant to clinical trials. This form attests to the absence of financial interests, or discloses the nature of any financial arrangements.
An Applicant May Consult With Fda As To Which Clinical Studies Constitute “Covered Clinical Studies” For Purposes Of Complying With Financial.
In compliance with the u.s. Fill out a separate financial disclosure form for each entity that could be considered a sponsor for this study.