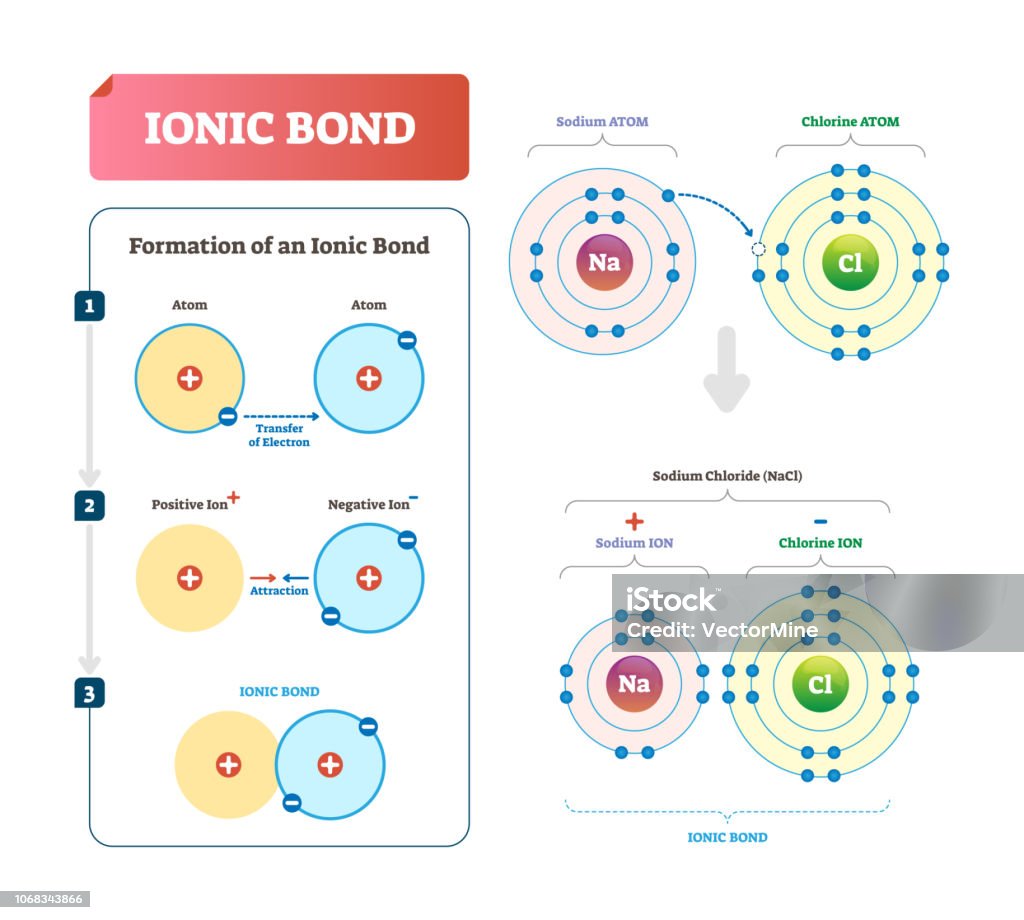
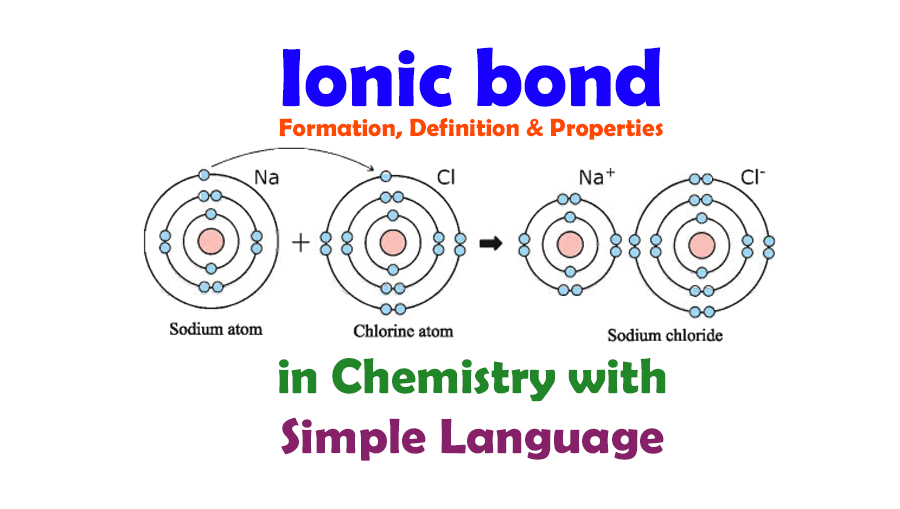
How Do Ionic Bonds Form - Only covalent bonding creates what we call a molecule. Ionic bonds involve a transfer of electrons between atoms of different elements. Ionic bonds form crystals, and metallic bonds form metals or metal alloys. Yes, atoms of copper and iron can generally form. A polyatomic ion is charged and will form ionic bonds;
Yes, atoms of copper and iron can generally form. Only covalent bonding creates what we call a molecule. Ionic bonds form crystals, and metallic bonds form metals or metal alloys. Ionic bonds involve a transfer of electrons between atoms of different elements. A polyatomic ion is charged and will form ionic bonds;
A polyatomic ion is charged and will form ionic bonds; Ionic bonds form crystals, and metallic bonds form metals or metal alloys. Only covalent bonding creates what we call a molecule. Ionic bonds involve a transfer of electrons between atoms of different elements. Yes, atoms of copper and iron can generally form.
Understanding Types of Chemical Bonds TEAS NurseHub
Ionic bonds involve a transfer of electrons between atoms of different elements. Only covalent bonding creates what we call a molecule. Yes, atoms of copper and iron can generally form. A polyatomic ion is charged and will form ionic bonds; Ionic bonds form crystals, and metallic bonds form metals or metal alloys.
Ionic Bond Vector Illustration Labeled Diagram With Formation
Only covalent bonding creates what we call a molecule. Ionic bonds involve a transfer of electrons between atoms of different elements. Ionic bonds form crystals, and metallic bonds form metals or metal alloys. A polyatomic ion is charged and will form ionic bonds; Yes, atoms of copper and iron can generally form.
ionic bond Definition, Properties, Examples, & Facts Britannica
A polyatomic ion is charged and will form ionic bonds; Ionic bonds form crystals, and metallic bonds form metals or metal alloys. Only covalent bonding creates what we call a molecule. Ionic bonds involve a transfer of electrons between atoms of different elements. Yes, atoms of copper and iron can generally form.
Chapter 7 Ionic Bonding. ppt download
Yes, atoms of copper and iron can generally form. Ionic bonds involve a transfer of electrons between atoms of different elements. Only covalent bonding creates what we call a molecule. A polyatomic ion is charged and will form ionic bonds; Ionic bonds form crystals, and metallic bonds form metals or metal alloys.
Ionic Bond Definition and Examples
A polyatomic ion is charged and will form ionic bonds; Ionic bonds form crystals, and metallic bonds form metals or metal alloys. Ionic bonds involve a transfer of electrons between atoms of different elements. Only covalent bonding creates what we call a molecule. Yes, atoms of copper and iron can generally form.
chemical bonding Ionic and covalent compounds Britannica
Yes, atoms of copper and iron can generally form. A polyatomic ion is charged and will form ionic bonds; Ionic bonds form crystals, and metallic bonds form metals or metal alloys. Ionic bonds involve a transfer of electrons between atoms of different elements. Only covalent bonding creates what we call a molecule.
What are Ionic Compounds and how they are formed?
A polyatomic ion is charged and will form ionic bonds; Ionic bonds form crystals, and metallic bonds form metals or metal alloys. Ionic bonds involve a transfer of electrons between atoms of different elements. Yes, atoms of copper and iron can generally form. Only covalent bonding creates what we call a molecule.
Ionic bond Definition, Properties, Examples, & Facts Britannica
Ionic bonds form crystals, and metallic bonds form metals or metal alloys. A polyatomic ion is charged and will form ionic bonds; Only covalent bonding creates what we call a molecule. Ionic bonds involve a transfer of electrons between atoms of different elements. Yes, atoms of copper and iron can generally form.
Ionic bond Definition, Properties, Examples, & Facts Britannica
Only covalent bonding creates what we call a molecule. Ionic bonds involve a transfer of electrons between atoms of different elements. Yes, atoms of copper and iron can generally form. Ionic bonds form crystals, and metallic bonds form metals or metal alloys. A polyatomic ion is charged and will form ionic bonds;
Ionic Bond Definition, Properties, Examples, Facts, 40 OFF
Ionic bonds form crystals, and metallic bonds form metals or metal alloys. Ionic bonds involve a transfer of electrons between atoms of different elements. A polyatomic ion is charged and will form ionic bonds; Only covalent bonding creates what we call a molecule. Yes, atoms of copper and iron can generally form.
Yes, Atoms Of Copper And Iron Can Generally Form.
Ionic bonds involve a transfer of electrons between atoms of different elements. Ionic bonds form crystals, and metallic bonds form metals or metal alloys. Only covalent bonding creates what we call a molecule. A polyatomic ion is charged and will form ionic bonds;