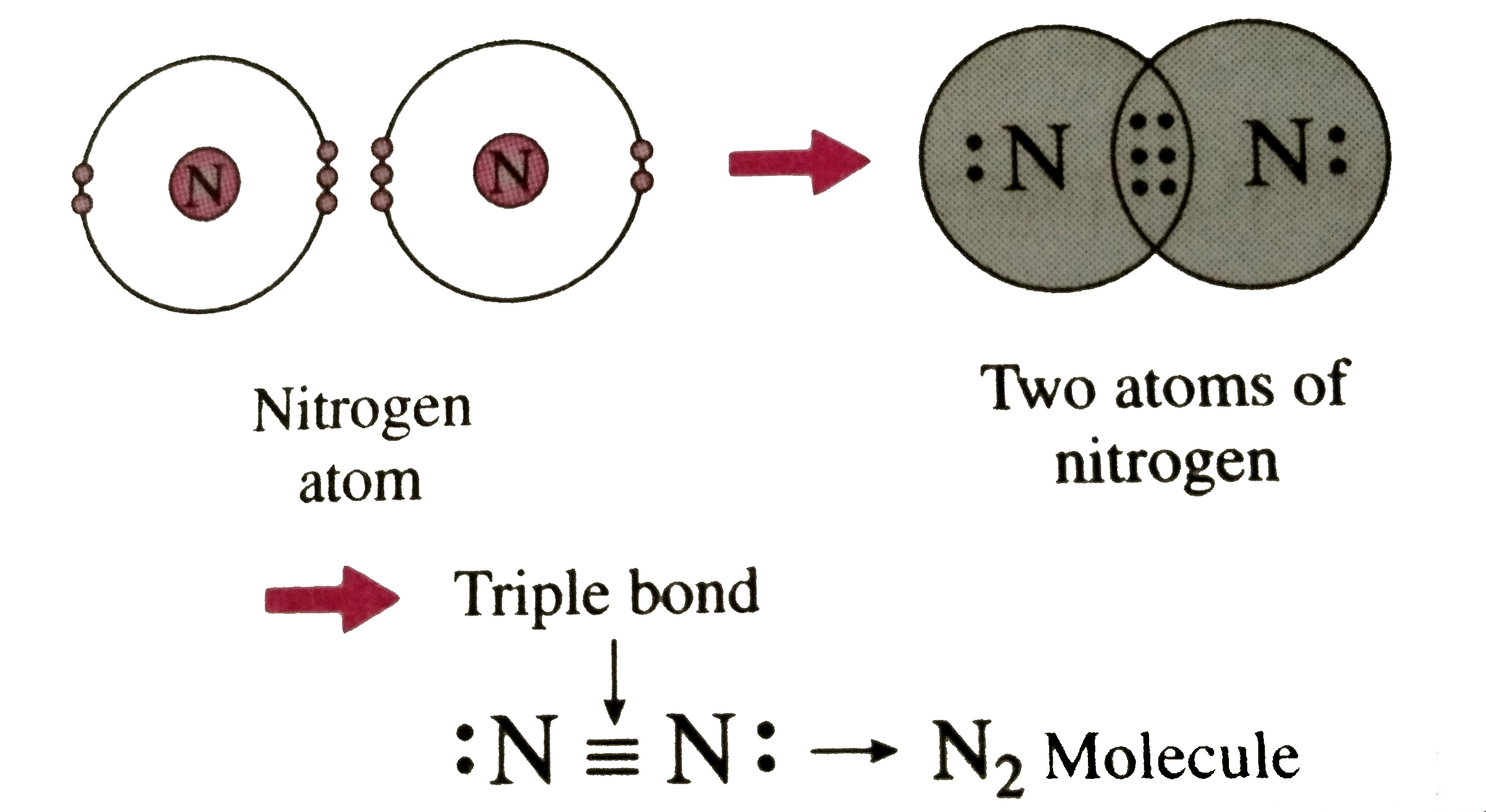How Many Bonds Can Nitrogen Form - Nitrogen is in group 5 and therefoe has 5 outer electrons. Nitrogen can form 3 covalent bonds and 1 coordinate bond. Would nitrogen and phosphorus form a covalent bond? Ammonia is a nitrogen atom bonded to three hydrogen atoms. That means it needs 3 bonds. How many covalent bonds are in ammonia? How many covalent bonds does water have? As known, nitrogen could form 3 bonds based on octet rule, because it has 5 valence electrons. There is a total of three covalent. No, nitrogen and phosphorus would not typically form a covalent bond with each.
Would nitrogen and phosphorus form a covalent bond? Water molecules have two simple covalent bonds between one oxygen and two hydrogen. How many covalent bonds are in ammonia? That means it needs 3 bonds. Ammonia is a nitrogen atom bonded to three hydrogen atoms. No, nitrogen and phosphorus would not typically form a covalent bond with each. There is a total of three covalent. How many covalent bonds does water have? Nitrogen can form 3 covalent bonds and 1 coordinate bond. As known, nitrogen could form 3 bonds based on octet rule, because it has 5 valence electrons.
Nitrogen can form 3 covalent bonds and 1 coordinate bond. There is a total of three covalent. How many covalent bonds are in ammonia? Ammonia is a nitrogen atom bonded to three hydrogen atoms. No, nitrogen and phosphorus would not typically form a covalent bond with each. How many covalent bonds does water have? Water molecules have two simple covalent bonds between one oxygen and two hydrogen. Would nitrogen and phosphorus form a covalent bond? As known, nitrogen could form 3 bonds based on octet rule, because it has 5 valence electrons. That means it needs 3 bonds.
PPT COVALENT BONDING PowerPoint Presentation, free download ID5128236
How many covalent bonds does water have? No, nitrogen and phosphorus would not typically form a covalent bond with each. There is a total of three covalent. How many covalent bonds are in ammonia? That means it needs 3 bonds.
Chapter 14 Ionic Bonds 2 41.49/50 = 82.98 3 37.93/50 = 75.86 ppt
No, nitrogen and phosphorus would not typically form a covalent bond with each. Would nitrogen and phosphorus form a covalent bond? As known, nitrogen could form 3 bonds based on octet rule, because it has 5 valence electrons. Nitrogen is in group 5 and therefoe has 5 outer electrons. How many covalent bonds are in ammonia?
PPT Covalent Bonding and Chemical Bonds PowerPoint Presentation, free
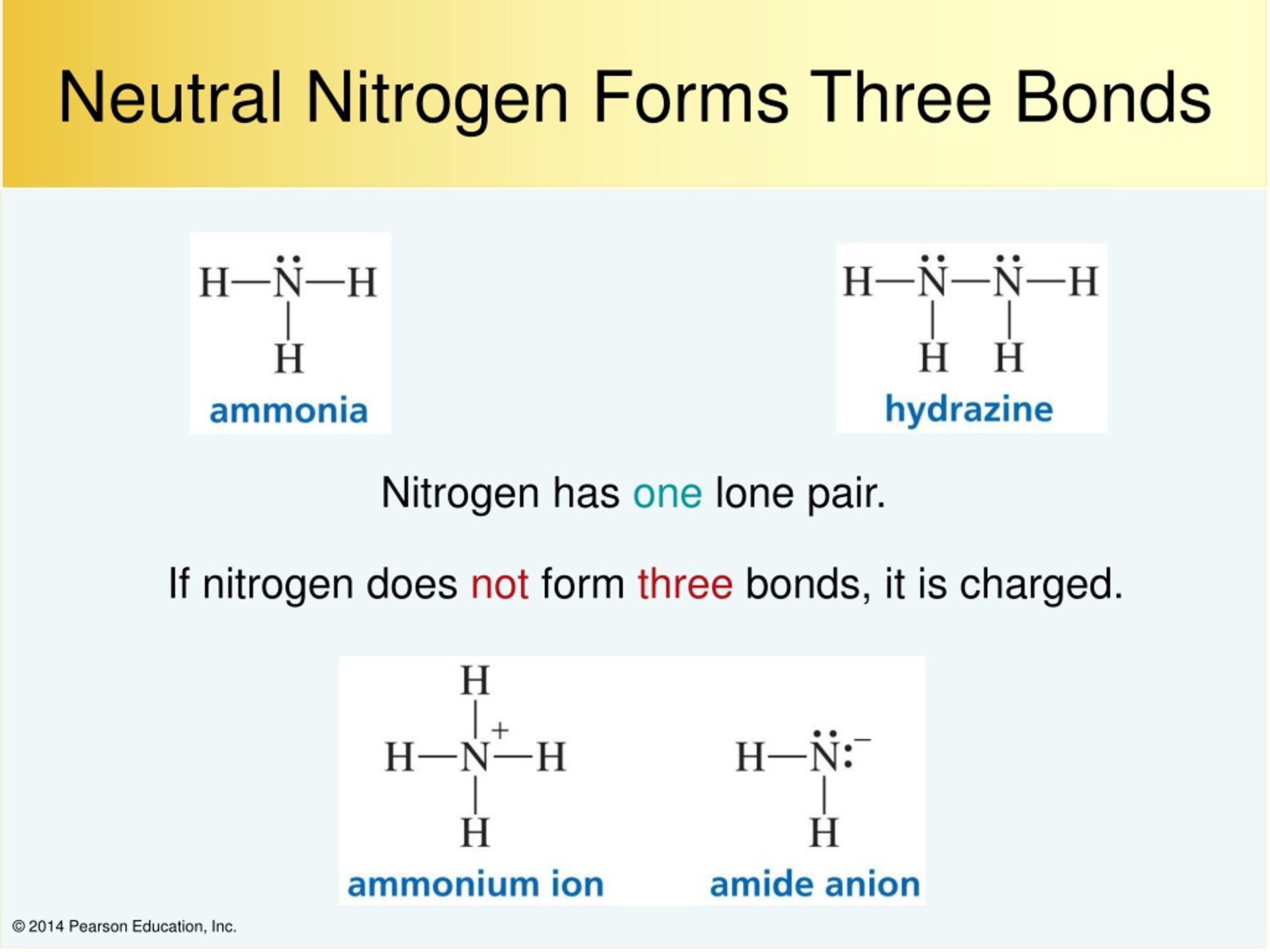
Ammonia is a nitrogen atom bonded to three hydrogen atoms. Would nitrogen and phosphorus form a covalent bond? As known, nitrogen could form 3 bonds based on octet rule, because it has 5 valence electrons. That means it needs 3 bonds. Water molecules have two simple covalent bonds between one oxygen and two hydrogen.
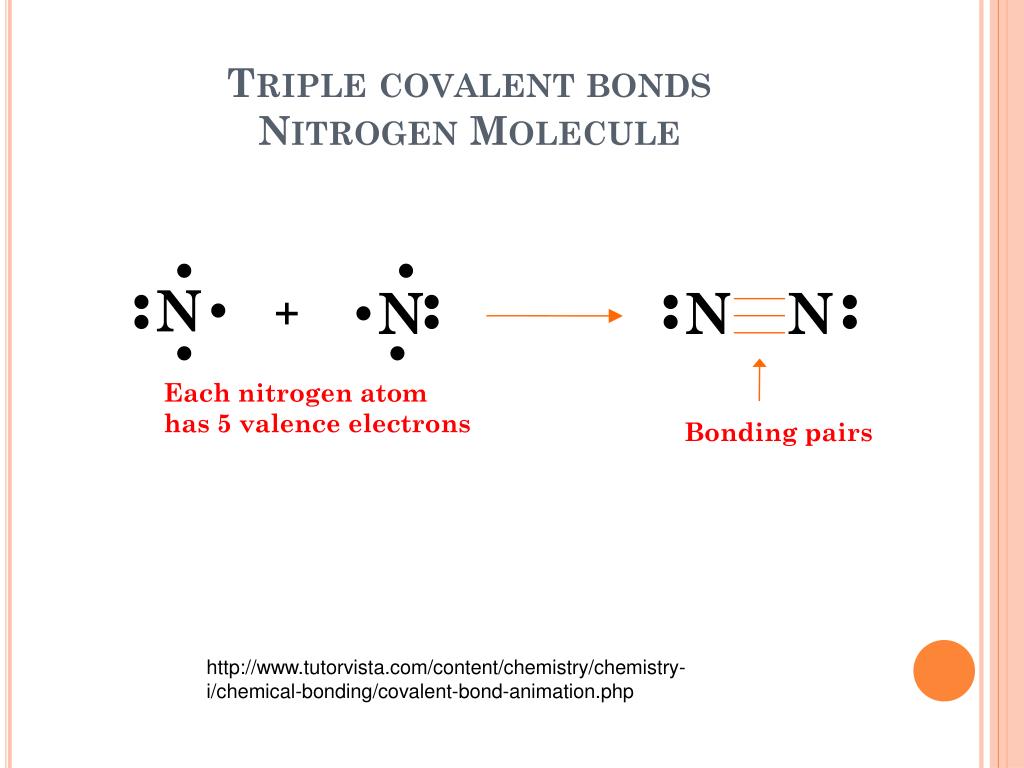
Nitrogen Covalent Bond
No, nitrogen and phosphorus would not typically form a covalent bond with each. Nitrogen can form 3 covalent bonds and 1 coordinate bond. There is a total of three covalent. How many covalent bonds does water have? Water molecules have two simple covalent bonds between one oxygen and two hydrogen.
COVALENT BONDING ORBITALS ppt download
Ammonia is a nitrogen atom bonded to three hydrogen atoms. Would nitrogen and phosphorus form a covalent bond? Nitrogen can form 3 covalent bonds and 1 coordinate bond. There is a total of three covalent. That means it needs 3 bonds.
Noble Gases. ppt download
No, nitrogen and phosphorus would not typically form a covalent bond with each. Would nitrogen and phosphorus form a covalent bond? How many covalent bonds does water have? Water molecules have two simple covalent bonds between one oxygen and two hydrogen. Nitrogen can form 3 covalent bonds and 1 coordinate bond.
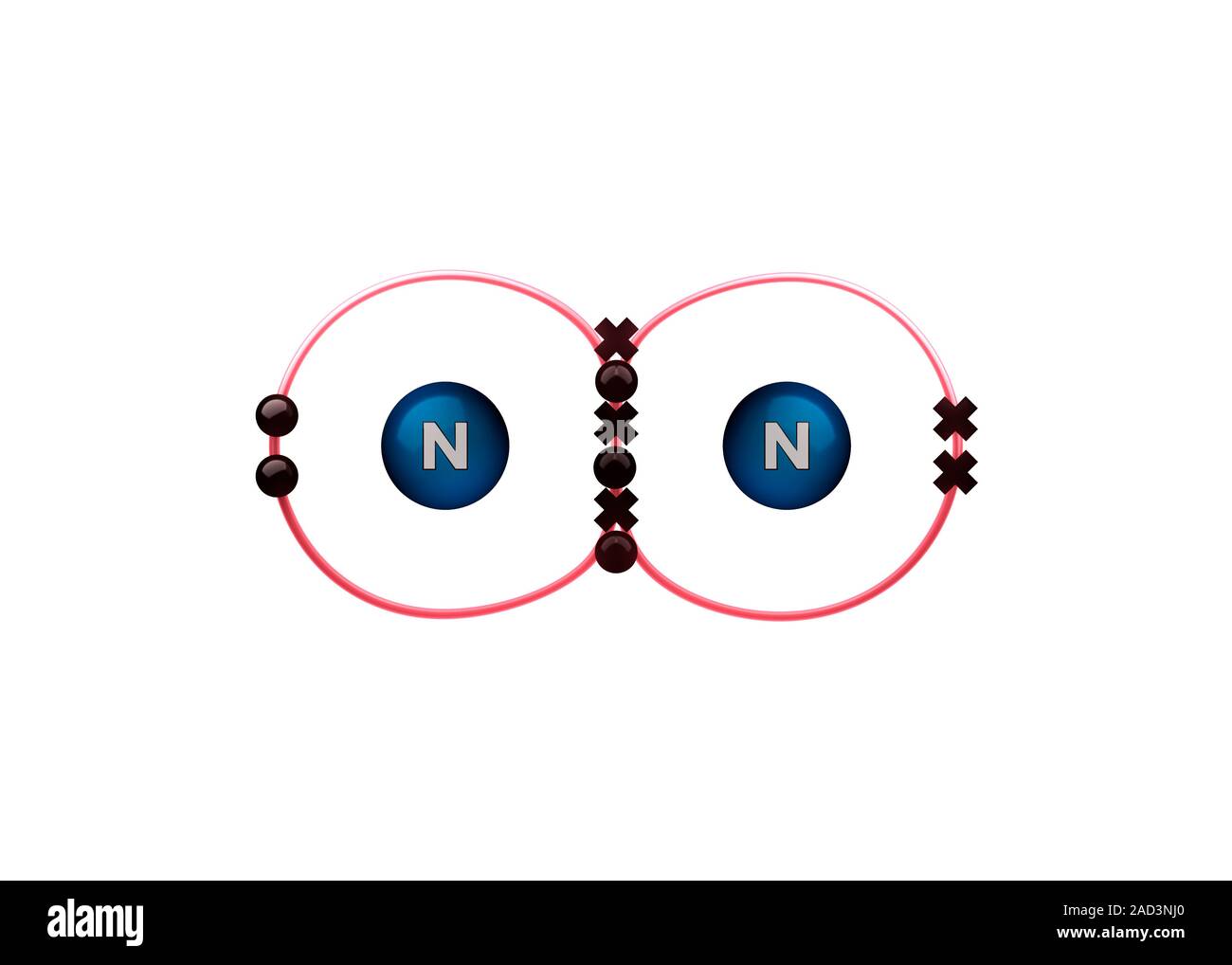
Bond formation in nitrogen molecule. Illustration of the sharing of
How many covalent bonds does water have? No, nitrogen and phosphorus would not typically form a covalent bond with each. Would nitrogen and phosphorus form a covalent bond? Ammonia is a nitrogen atom bonded to three hydrogen atoms. There is a total of three covalent.
Triple Bond Nitrogen
There is a total of three covalent. Would nitrogen and phosphorus form a covalent bond? Nitrogen is in group 5 and therefoe has 5 outer electrons. No, nitrogen and phosphorus would not typically form a covalent bond with each. Nitrogen can form 3 covalent bonds and 1 coordinate bond.
The Nitrogen Cycle. ppt download
No, nitrogen and phosphorus would not typically form a covalent bond with each. Water molecules have two simple covalent bonds between one oxygen and two hydrogen. That means it needs 3 bonds. Nitrogen is in group 5 and therefoe has 5 outer electrons. How many covalent bonds does water have?
PPT Remembering General Chemistry Electronic Structure and Bonding
No, nitrogen and phosphorus would not typically form a covalent bond with each. As known, nitrogen could form 3 bonds based on octet rule, because it has 5 valence electrons. Water molecules have two simple covalent bonds between one oxygen and two hydrogen. Ammonia is a nitrogen atom bonded to three hydrogen atoms. There is a total of three covalent.
Water Molecules Have Two Simple Covalent Bonds Between One Oxygen And Two Hydrogen.
Would nitrogen and phosphorus form a covalent bond? That means it needs 3 bonds. How many covalent bonds does water have? How many covalent bonds are in ammonia?
Ammonia Is A Nitrogen Atom Bonded To Three Hydrogen Atoms.
Nitrogen is in group 5 and therefoe has 5 outer electrons. Nitrogen can form 3 covalent bonds and 1 coordinate bond. No, nitrogen and phosphorus would not typically form a covalent bond with each. There is a total of three covalent.